About the lab
The goal of the Hollern lab is to study tumor-immune cell interactions and to use this information to enable the development of treatment approaches optimizing immune cell identification, elimination, and control of cancer cells in the body.
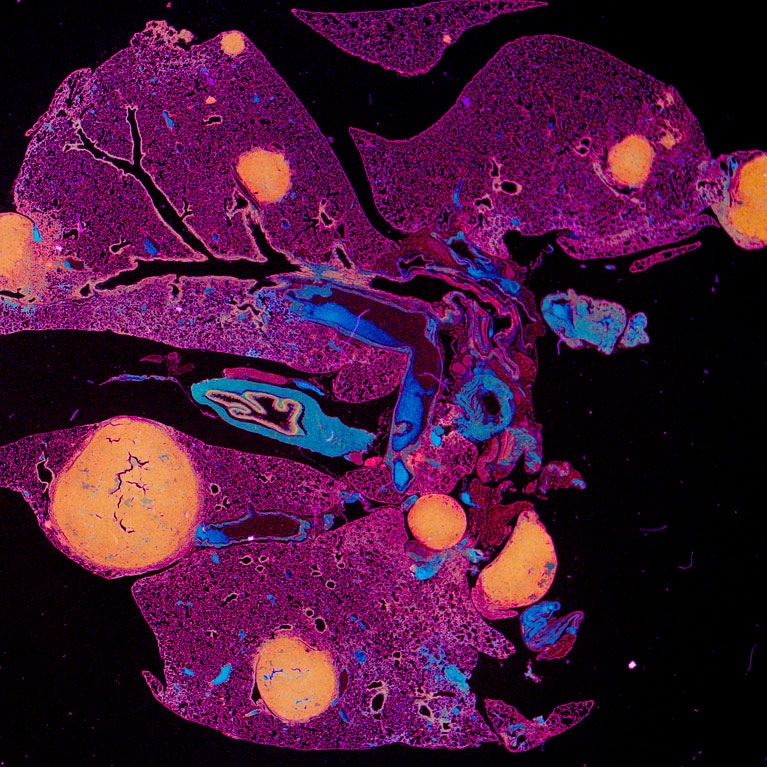
Similar to the immune system’s protection against pathogens, it also safeguards tissues from becoming overridden by oncogenesis. Gaining an understanding of molecular regulation of immune cell activity in cancer has allowed for the development of therapeutic antibodies, classified as immune checkpoint inhibitors, which invigorate immune cell targeting and cytotoxicity within cancer cells. For example, immune checkpoint inhibitor antibodies to PD-1 or PD-L1 can reprogram T cells (a type of immune cell) to expand and kill cancer cells. While these therapies are revolutionizing outcomes for some cancers, they are in dire need of improvement in order to better help patients with advanced solid tumors. For example, in triple negative breast cancer, patient response rates to immune checkpoint inhibitors are low, hovering around 10%-20%.
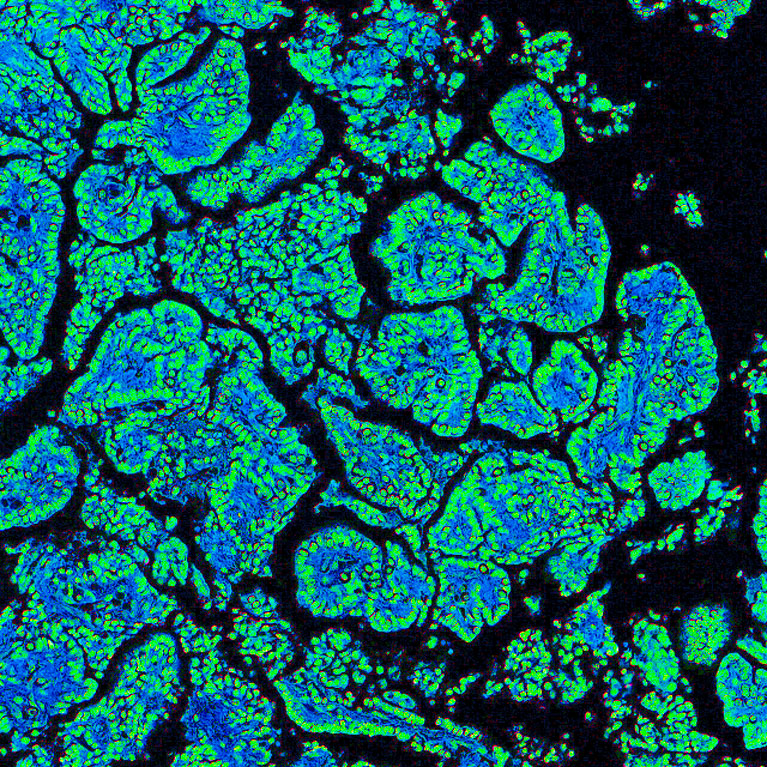
The Hollern Lab has found that B cells represent a key cellular regulator of the anti-tumor immune response to immune checkpoint therapy. Importantly, we have found that patients who harbor tumor infiltrating B cells have a higher likelihood of responding to chemotherapy and immunotherapy. With experimental models, we provided the first report that the efficacy of immune checkpoint inhibitors and anti-tumor immunity are highly dependent on the activity of B cells.
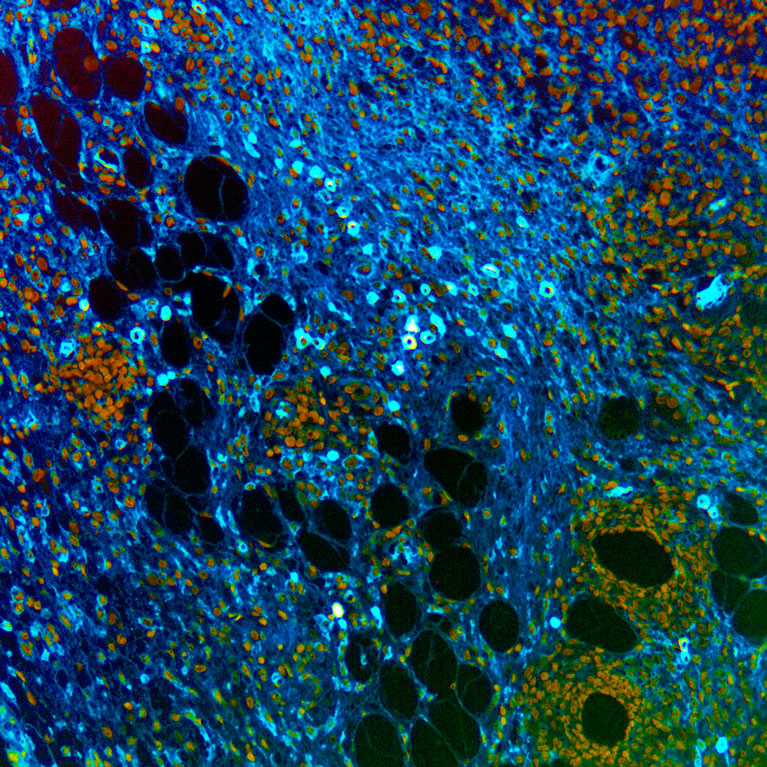
From this work, we learned that B cells have the phenomenal potential to control anti-tumor immunity and to improve current response rates to immune checkpoint therapy. Our mechanistic studies show that B cell responses to immune checkpoint therapy in the primary tumor are dependent upon CD4+ T cells and IL21. In turn, B cells co-activate T cells and drive their anti-tumor immune function. Interestingly, B cell activation by CD4+ T cells appears to drive the formation of plasma cells, a type of B cell that is capable of secreting antibodies which can bind and lead to the elimination of tumor cells. In our experimental models, we find evidence that these antibodies are specific to tumor cells and are required for curative responses to immune checkpoint inhibitors. These findings B cells a key cellular regulator of the anti-tumor immune response; potentially connecting adaptive and innate immunity. As a result, we are now testing, studying, and searching for novel mechanisms to control and leverage the ability of B cells to drive the eradication of cancer therapeutically.